LABS FOR LIFE (L4L) PROJECT
Labs for Life is a partnership initiative between MoHFW& the U.S. Centers for Disease Control and Prevention (CDC) with the objectives ofimprovingquality of laboratory services and buildingsustainable laboratory systems within the public health sector. The project is being implemented through Christian Medical Association of India (CMAI).
The project is being implemented in two phases.
PROJECT PHASE I
Phase I is demonstrative in its large objective. It was launched on 4th December 2014 in 20 selected facilities (10 each in tertiary and secondary care) in 10 districts across the 6 States in 5 regions.
Specific objectives of the Phase 1 are to:
- Enhance capacity for diagnosis of communicable and non-communicable diseases in India
- Improve the quality of services of the 20 identified labs, from the baseline assessment, by at least two additional stars/grades/levels as measured by the checklist
- Strengthen specimen referral mechanisms and linkages between various levels of facilities,
- Ensure sustainability of interventions through local, state-level and country ownership
Key activities of the project so far,
Project Organization and Governance: As a first step, the Labs for Life (L4L) project defined the project governing mechanism and developed a structure to spearhead the project activities. At the National level, the core committee under the chairmanship of Additional Secretary, MoHFW is responsible for review, advice and provision of strategic direction to the project. The committee consists of representatives from MoHFW, CDC, BD, CMAI, National Health System Resource Center and National Health Mission. The committee meets once in a quarter and seven core committee meetings have been conducted so far.
At the state level, the Principal Secretary-Health, Mission Director-National Health Mission, Director of Health Services, Director of Medical Education, State Program Managers, State Quality Assurance Officer are among the officials briefed and updated on the project activities. At the district level, District Health Officer, Chief Medical & Health Officer, District quality assurance unit, district program manager are the key stakeholders involved in the planning and implementation of the project. At the Institutional Level, the Principal, Dean, Medical Superintendent, Nodal officers, Head of the laboratories and other concerned officers are involved in the planning and implementation of project activities.
Baseline Assessment, Gap Analysis and State level Dissemination: The objectives of the assessment was to understand the existing laboratory practices, identify areas of gaps/deficiencies, emerging challenges, document best practices, and to decide on the strategies and interventions for implementing quality management systems. All selected laboratories under the project were included in the assessments, conducted from 23rd March to 10th April 2015.
Baseline Gap Analysis - National Report 2015
Following the finalization of reports, the findings were disseminated in all the states, with the objective of bringing together all the stakeholders, jointly plan and develop strategies to address the gaps.
Capacity Building: Based on the situation assessment and gap analysis, followed by series of consultation meetings, the key systemic areas of concern that could be addressed through capacity building were finalized. These included Facility Management and Safety, Sample Collection, Documentation, Equipment Management, Calibration and Controls, Testing Methodologies, Inventory Control, Staff Training Practices, Setting and Monitoring Quality Indicators and Usage of Information Technology. A comprehensive capacity building plan with central, district and facility wise trainings was developed to guide the planned activities. The plan consists of, Training of Trainers (ToT), district level onsite training, handholding by Regional Quality Consultants, Periodic mentoring visits by the L4L central team, and e-learning initiatives such as webinars. In addition regular advocacy with all stakeholders is being done.
Training Modules: Several modules on technical areas, such as, Sample Collection and Pre-Analytical Best Practices, Documentation and Record Management, Facility Management and Safety, Equipment Management and Quality Control (2 volumes), Quality Management System (Management Requirements), Inventory Control, Post Analytical Best Practices have been developed so far, and extensively being used by the participating institutions. Many of these are being vetted by National Health Mission.
1. Draft Sample Collection Module
2. Draft Documentation Module
3. Guideline document for design of BSL 2 Labs Level
4. Training Module on Management Requirements
5. Training Module on Quality Control
Training of Trainers: National level workshops on Sample Collection and Pre-Analytical Best Practices, Facility Management and Safety, Equipment Management, Documentation and Quality Control were conducted. The objective was to train a pool of Master Trainers who can go back and train their co-workers in their institutions.
Onsite Training: Onsite training on “Sample Collection and Pre-Analytical Best Practices, Facility Management and Safety, Equipment Management, Documentation and Quality Control” are regularly being conducted. Technical staff from national programs was also included in the onsite training. On special requests from National Health Mission, a few people from other public health facilities were also trained in some locations. Further training are calendared to ensure a cascading process to reach all the laboratory technical staff of the identified institutions and if possible, beyond.
Webinars: Several webinars have been conducted. Technical topics such as, Lab Process Control, Calibrations, Documents and Records, IHR, Infection Control, Bio Medical Waste Management, Basic Microbiology Technics, Epidemiological Typing technics, Serology EQAhave been covered so far. On an average, around 75-100 participants attend the webinars from across the country.
Facility Specific Interventions:In addition to addressing the systemic challenges, facility specific interventions were also undertaken in terms of technical advice for lab renovations in several institutions, Bio Medical Waste Management issues etc.
Initiatives to tap the existing resources: A district and state level resource mapping was done by the Regional Quality Consultants to understand the current resources available for laboratory strengthening under various government programs and schemes. Though National health Mission has a mandate to address the infrastructure, human and financial gaps in the public health care system, laboratories have not gained adequate attention so far. To tap the existing available resources under NHM, the project facilitated developing Project Implementation Plan (PIP) in all states to address the resource gaps. As a result, allocations have been made in some states.
Website: Understanding the unmet need for in-service training for technicians, Labs for life is developing a website with learning resources like training modules, videos and self-learning platforms. Resources like e-tool for Quality Indicators and Statistical Quality Controls are being developed.
Consultation for Medical Colleges: During the course of the project, it was understood that there were no prescribed standards for service provision by Government Medical College Hospitals. Two consultations have been held towards this with representation from MCI, DME, DGHS, and the Medical Colleges and subcommittees were formed to develop recommendations for staff, equipment menu, testing menu and POCT in medical college laboratories. A draft report is being readied for further action.
Mid-term Assessment
A Mid-term review was conducted at L4L sites in Sep-Oct 2016 to assess the progress towards project objectives and to identify thrust areas for guiding further activities. In all, 16 facilities (8 each in tertiary and secondary care) were covered in this review, by independent, external assessors using the same assessment tool that was used for baseline assessment. The specific objectives of the mid-term review were to:
- Understand the progress, gaps and challenges and the factors facilitating or hindering the progress/achievements.
- Document lessons learned and challenges.
- Formulate facility-specific strategic plans for taking corrective measures and to improve quality management systems.
1. Gap Analysis - Mid Term Review
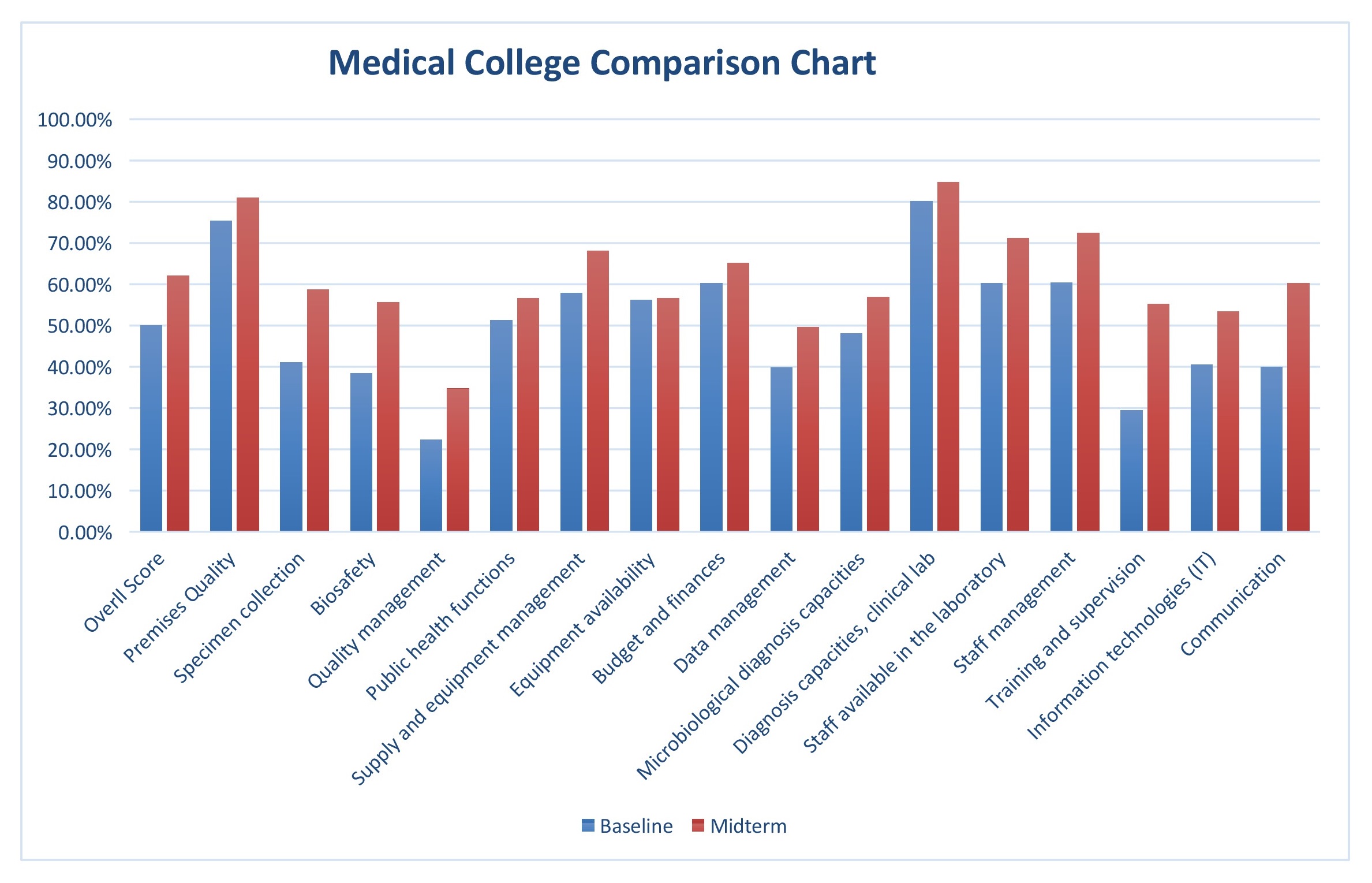
Medical Colleges: All medical colleges registered an improvement compared to baseline scores. Appreciation was more for certain elements viz. Specimen collection and handling, Biosafety, Staff management and availability, training and supervision, use of IT, and Communication. It was marginal to moderate for others i.e. Premises quality, Public health functions, Equipment availability and management, Budget and finances, Diagnostic capacities etc.
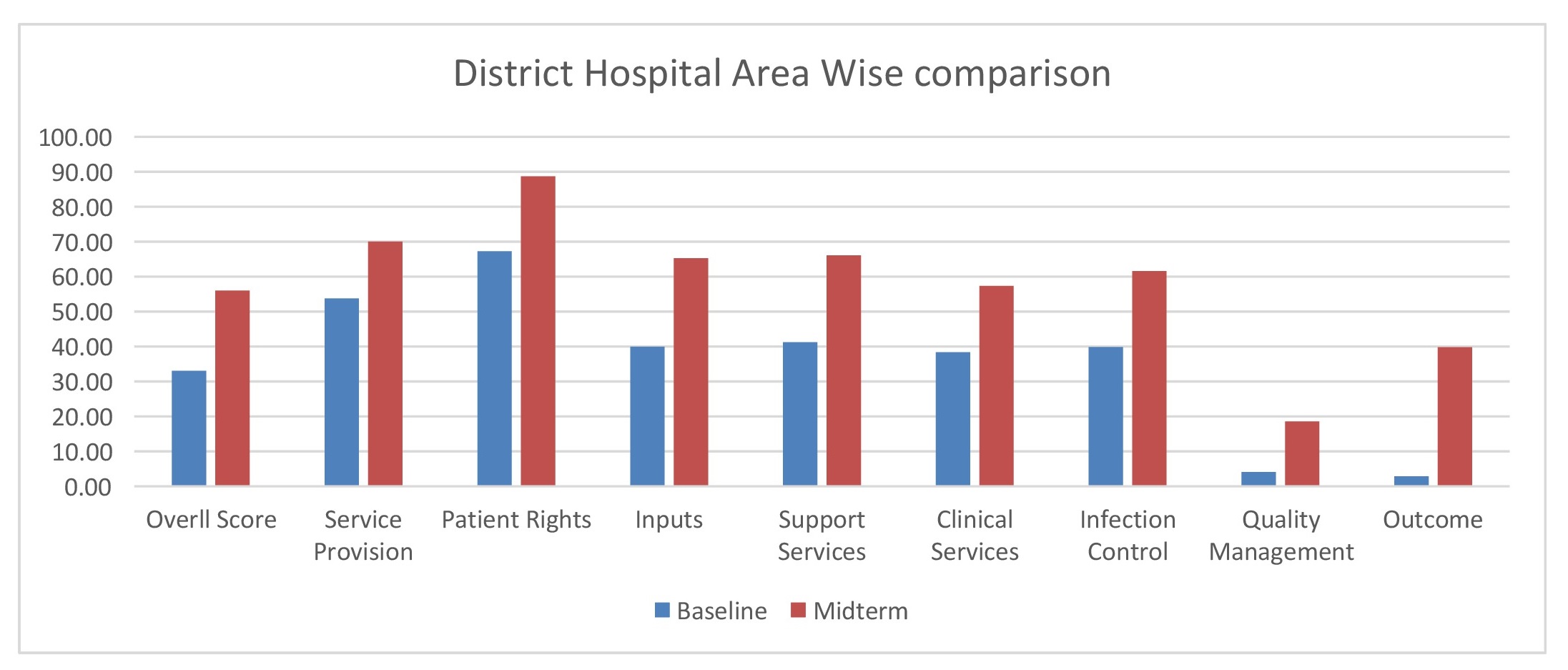
District Hospitals:In District hospitals, a cross-sectoral improvement was observed. Improvement was maximum for Outcomes, Quality management, Inputs, Support services, Infection control, Clinical services, Patient rights and Service provision, in that order.
National effort for NABL accreditation: In view of promising outcomes from the early project activities, the objectives were changed from achieving improvement in lab services – in terms of capacity, quality and sustainability; to achieving accreditation for conformance to the ISO15189 standard for selected laboratories from amongthe L4L project participants. Nine labs have been selected for starting the accreditation process. Continuous Training and close mentoring support are being provided to these labs. This includes Internal Auditor training for identified laboratory Quality Managers, additional support through handholding to complete the QMS documentation, arranging mock internal audits through L4L. NQAS certification is also being encouraged for those facilities who are partially ready.
Achievements of the project
Primary Objectives
1. Enhancing Diagnostic Capacities:
- Expansion of scope of testing has been encouraged in all facilities. Free Diagnostic Initiative menu has been advocated for secondary care centers and many have started working towards it. Examples:
- Biochemistry testing menu was expanded in AMC Vizag (following procurement of fully automatic analyzer) and at Dr. N.T.R. Area Hospital, Annakapalle.
- At District Hospital, Wardha, serum electrolytes and lipid profile were added to the test menu
- Pap smear reporting as per Bethesda system started in many hospitals
2. Improving the Quality of Services:
- Workforce development through training and handholding
- Internal Quality Controls has started in several labs
- EQAS registration of several labs initiated
- Infection control practices like use of evacuated tubes, disposable containers, and segregation of biomedical waste at sourcestarted in all medical colleges.
- Equipment management – IQ (Installation Qualification), OQ (Operational Qualification), PQ (Performance Qualification) for many newly installed lab equipment done and documented. Maintenance and calibration practices for equipment being streamlined.
- Documentation of QMS being completed in several labs
- 9 labs with demonstrated interest and commitment have been selected for NABL Accreditation process in the near term
3. Strengthening specimen referral mechanisms and linkages between different levels:
- Draft guidelines prepared and submitted to NHM for vetting
4. Ensuring sustainability of interventions through ownership
- Funds were mobilized from NHM through the PIP route for Rajasthan, Telangana, Maharashtra and Assam facilities.
- Advocacy being undertaken for resource mobilization and inclusion of all stakeholders in the improvement process
- Measures of set standards for service provision in medical colleges in progress
- Central Collection Facility was set up following dissemination at Assam Medical College, Dibrugarh,
- Space was allocated for Central Lab in NBMC, Siliguri, AMC, Vizag, GMC Aurangabad, RNT Medical College, Udaipur and SNMC, Jodhpur. Construction work is completed at AMC, Vizag where the lab is functional, while it is ongoing at the other facilities.
- New equipment, both analytic and non-analytic, were procured in several facilities e.g. Fully automatic analyzer in Dept. of Biochemistry, AMC, Vizag, as well as GMC, Aurangabad
- Biomedical Waste Plant was set up in Assam Medical College, Dibrugarh, following trainings,
Other achievements
•Sharing of best practices
- Labs for Life was selected for a poster presentation on National Summit on Good, Replicable and Best Practices in Public Health Care in India at Tirupation 31st Aug, 2016.
- The case study of District Hospital Paota, Jodhpur was published in the 4th NHM Coffee Table book on good and replicable practices and innovations in public healthcare systems in India. Another case studyfrom Andhra Medical College, Vizaghas been communicated to the government.
• Developing training and e-learning resources
- The L4L project has developed a website which was launched in the month of January 2017. The website serves as a resource repository on lab related processes.
- It has FAQs on various lab aspects.
- Several training modules on technical and quality areas of the laboratory have been developed and uploaded on the website where they are freely accessible.
- Other learning resources like videos and PPTs are in the pipeline, the first set of videos (dealing with Infection Control) were released on 25th Sep, 2017
•Technology and Innovations
- The L4L website also carries management e-tools which enable tracking of Key Performance Indicator (KPIs) with regard to Quality.
- Statistical Quality Control tool software is also available at the website. This tool is jointly developed with CMC Vellore for the laboratory staff to help them in laboratory statistical calculation. This will provide the handy date and speedy calculation of Lab statistics.
•LIFE Initiative – Laboratory Improvement for Excellence:
Taking forward the learning from Labs for Life, NHM has launched the LIFE initiative on 6th July, 2017during the 4th National Summit on Good, Replicable and Best Practices in Public Health Care in India at Indore with the objectives of:
- Facilitating the compliance of District Hospital laboratories to IPHS standard
- Building capacities of the District Hospital laboratories to perform the of tests listed in theFree Diagnostic Services Initiative
- Enabling compliance of the laboratories to ISO standards as evidenced by 1. Certification from National Quality Assurance Standards (NQAS) 2. Accreditation by National Accreditation Board for Testing and Calibration Laboratories (NABL).
- The Initiative offers to help states and institutions to understand the gaps in their laboratory systems& provide mechanisms for training and workforce development and to Establish Laboratory Quality Management Systems in compliance with the ISO standard.
While phase I is still continuing and will be over in nearly one year simultaneously phase II has been initiated.
LABS FOR LIFE PROJECT PHASE II
- Phase II of the project has been envisaged to strengthen public health laboratories in institutions housing ART Centers. This is to ensure that the laboratory support for HIV patients, as prescribed in the NACO operational guidelines, is easily available at the point of care and is demonstrated to meet internationalstandards of laboratory services. To that end, learning from Phase I, in terms of project layout as well as the specific strategies for establishing quality management systems in clinical laboratories would be implemented in the Phase II.
- This phase has been launched in 22 selected ART center-Hospitals across 6 districts in two states – Maharashtra and Andhra Pradesh.
The specific objectives of Phase II are to:
- Bridge the gaps in supporting investigations required as per NACO Operational guidelines for ART Services and Free Diagnostics Service Initiative
- Establish Quality Management Systems in selected co-located district level laboratories with ART facility;
- Establish linkages between ART Centers and the public health laboratories;
- Develop mechanisms for detecting Opportunistic Infections (OIs)
Project Layout (2017-2019)
• Activity 1: Baseline Assessment and Gap Analysis - Gap Analysis - Andhra Pradesh, Gap Analysis - Maharashtra
A baseline assessment will be done in all facilities to reveal present gaps in capacity, quality and referral linkages; and identify facility-specific challenges and systemic areas of need that must be prioritized and addressed.
• Activity 2: Capacity Building Activities
In-service training for frontline, technical supervisory and top level managerial lab staff will be imparted through a mechanism of Training of Trainers (ToT) and onsite trainings. Innovative approaches for continuing education, such as the use of instructional videos and short online courses will be used.
• Activity 3: Implement Quality Management Systems
Mentoring and handholding will be provided through local support systems, field level staff and State Cluster Coordinators placed in each state to help the laboratories implement the quality management concepts gained during training. Supporting and mentoring visits will be conducted by the central team as required
• Activity 4: Addressing Infrastructure Gaps through Sensitization, Advocacy, and Resource Mobilization
Apart from technical inputs imparted through training and mentoring, wherever laboratory support mandates financial and human resource inputs, sensitization of higher management and resource mobilization through advocacy is envisaged. This will help bridge the gaps in supporting tests and also the implementation of quality management in labs.
• Activity 5: Strengthening Linkages between ART, ICTC and other labs
In line with its primary objective, the project will work with other development partners like Share and I-Tech to develop a system of linkages in order to ensure that patients reporting to ART Centers, ICTCs and other like labs get directed for all necessary supporting investigations to the main labs. The project will seek to enhance the coordination between SACS and state/municipalhealth/medical education departments and NHMfor full capacitation of this process.
• Activity 6: Periodic Reviews of the Cluster Activities
Periodic reviews will be undertaken using the baseline assessment as reference for guiding interventions or resetting their priority. This will be done in consensus with the stakeholders
Key activities of the project so far,
Project Organization and Governance: At the outset, a governing mechanism was defined to strategize, monitor and handhold the implementation of the project. At the National level, the core committee under the chairmanship of Deputy Director General (Lab Services), NACO is responsible for review, advice and provision of strategic direction to the project. The committee consists of representatives from MoHFW, NACO, NHM, CDC, BD, CMAI, Share India and I-TECH. It will meet at regular intervals to review the progress of the project and ensure that programmatic milestones are achieved as outlined in respective work plans.
At the state and district level, the activities are planned in consultation with all relevant stakeholders from State Health departments, State/District AIDS Control Societies, Institutions and development partners. Monitoring at the state level will be throughthe state cluster coordination mechanism. The district level activities will be monitored through DAPCU (District AIDS Program Control Unit).
Baseline Assessment, Gap Analysis and State level Dissemination: The objectives of the baseline assessment were to understand the existing lab structure and service provision vis-à-vis the mandated requirements as per NACO Guidelines; identify facility-specific as well as across the board challenges in service provision, quality management and referral linkages between ART centers and labs; and formulate strategic plan for establishing quality management systems in each facility.
To facilitate the assessment, it was preceded by a sensitization of the Nodal Officers nominated by the institutions to coordinate the lab improvement activities. The baseline assessment was conducted in all the 22 public health laboratories from 26th to 29th July 2017 using a validated assessment tool (Checklist no. 13) of National Quality Assurance Standards (NQAS) designed by NHM and modified to capture laboratory support and linkages for PLHIV. The institutions were scored on service provision, patient rights, inputs, support services, clinical services, infection control, quality management and outcome measurement, apart from referral linkages and their monitoring.
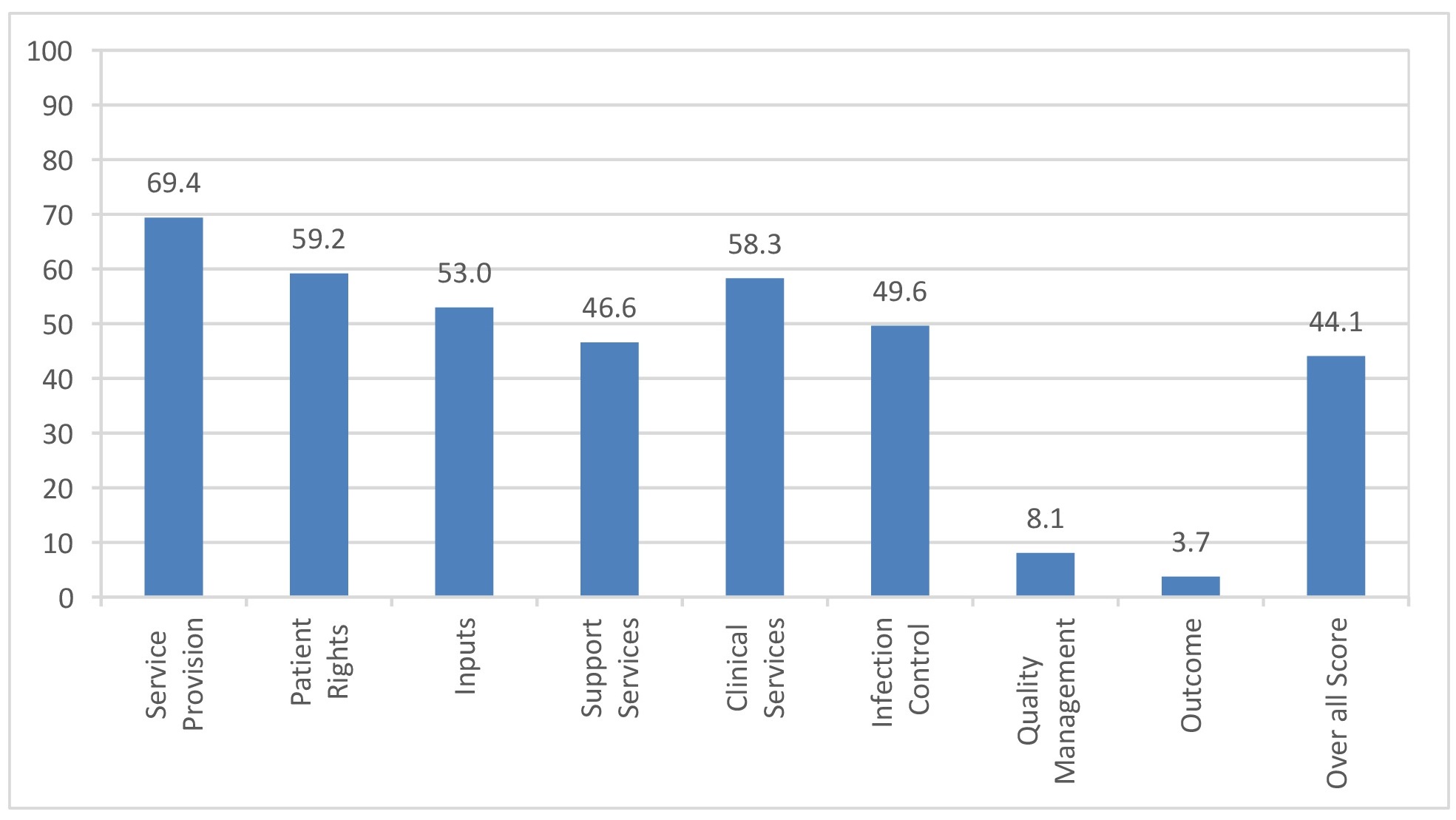
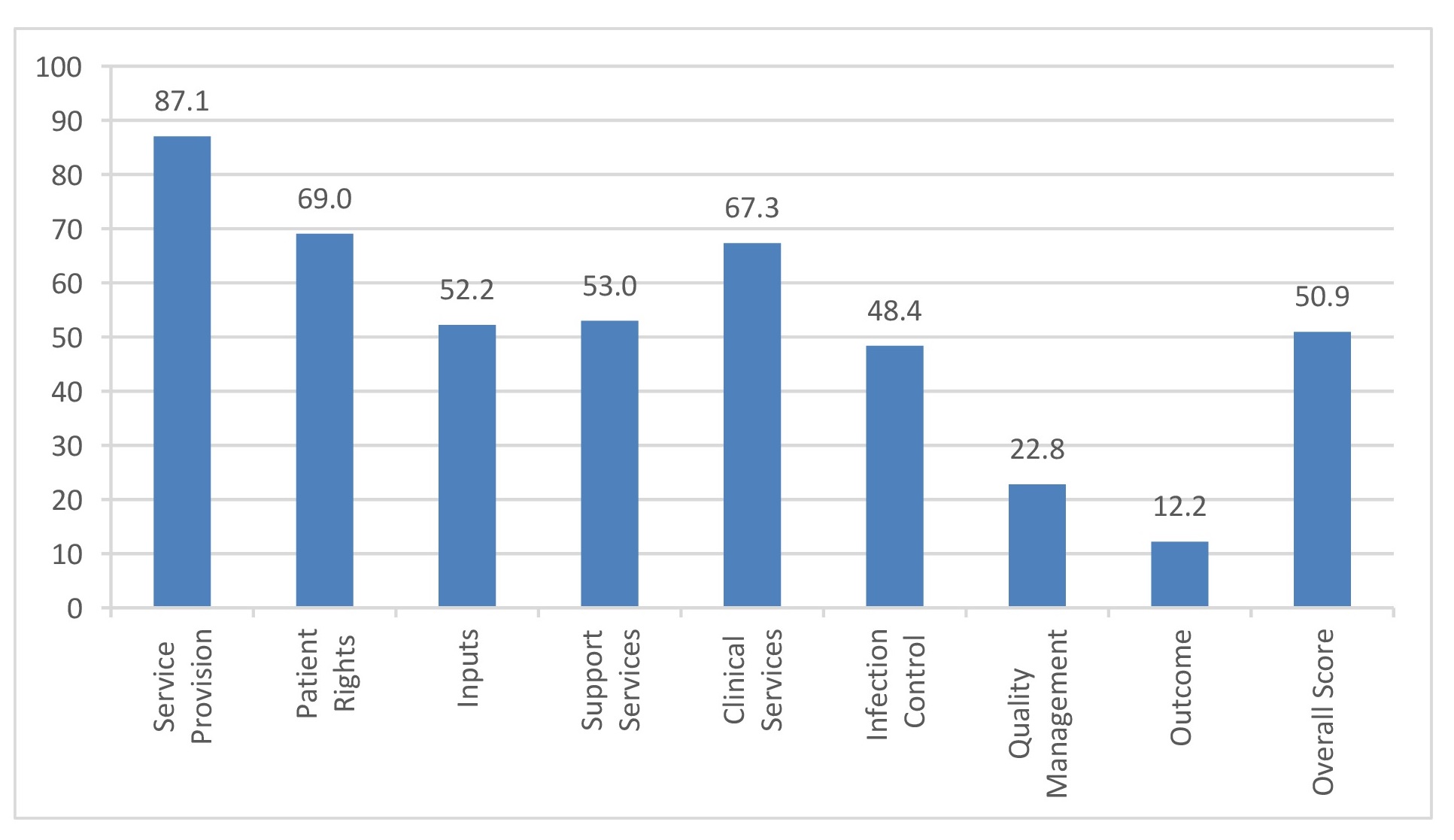
Key findings: The institutions scored maximum in provision of services with tertiary centers doing better than the district level/ corporation hospitals. The mean scores were near the half way mark for patient rights, inputs, support services, clinical services and infection control, with a few institutions doing better than the others. The mean and individual scores were uniformly poor across the institutions in areas of quality management and outcome measurement, barring few exceptions. Referral linkages were present and functional in most institutions, though there was ample scope for improvement. A major area of concern was the deficiency of microbiology services pertaining to screening for opportunistic infections, important in the follow up of PLHIV, at almost all secondary centers.
A. Overall Institutions Scores - Maharashtra – Cluster districts (Mumbai, Thane & Pune)
B. Overall Institutions Scores - Andhra Pradesh – Cluster districts –East Godavari, Krishna and Guntur
The findings have been disseminated to the stakeholders. The final report and action plan is being readied.
Capacity Building: Based on the situation assessment and gap analysis, ToTs have been held on Sample collection and pre-analytical best practices, and overview on Quality Management Systems. A workshop on Equipment management has been planned in the immediate future. Further training calendar would be developed according to the action plan and will go on in tandem with the on-site mentoring and handholding activities.
Training resources: The Phase II of the project isutilizing the resources developed during the Phase I towards enabling the institutions to establish a quality management system for the laboratories. These resources include training modules, web-based resources like instructional videos and webinars. These would supplement hands-on workshops and teaching sessions. Additional resources will be developed to cater to the specific objectives of Phase II. A manual on Microbiology is being developed for the District level hospitals to strengthen their capacities for microbiological tests including screening for opportunistic infections (OI).
Sensitization and Advocacy: The sensitization of nodal officers and heads of institutions to various resources, processes and continual improvement strategies has been undertaken before, during and after the baseline assessment. The action plan is being made through stakeholder participation and will be reinforced through frequent on-site visits and support.
Strengthening Linkages between ART, ICTC and public health laboratories: The assessment findings have been shared with the State/District AIDS Control Societies and state/municipal health/medical education departments. The action plan will include commitments from all stakeholders in order to improve the referral linkages and their monitoring.
Taken to its logical conclusion, this innovative, multi stakeholder project promises to enable the selected laboratories to fulfill the operational guidelines of NACO and provide laboratory support to PLHIV before and during ART.
This site will be periodically updated. Any suggestions or comments are welcome on labsforlife[dot]mohfw[at]gmail[dot]com